

Chromite is a dark grey, metallic, oxide mineral. Due to its metallic luster and occasional magnetic properties, chromite is often mistaken for magnetite. Chromite can often be found in basic igneous rocks, and in the metamorphic and sedimentary rocks created by rocks containing chromite that were affected by weather. A particularly useful metal, chromium, can be extracted from chromite ore.



| Color | Dark grey, black |
|---|---|
| Luster | Metallic |
| Streak | Brown |
| Hardness (Mohs) | 5.5 |
| Cleavage | None |
| Specific Gravity | 4.0 |
| Chemical Formula | FeC2O4 |
| Crystal Structure | Isometric |
| Classification | Oxide |
Chromium is used for making hard, tough, and chemical resistant steel. The alloys produced by chromium are commonly called “stainless steel”. They are particularly special alloys in that they are resistant to high temperatures, making them useful in kitchen appliances and engines. Chromium is used in lots of geothermal power systems because of its heat resistance and strength. Engineers coat the steel pipes that run through the ground in chromium alloys to ensure that the pipes are safe and stable while processing intense heat and pressure.



Chromite is the only ore of chromium, making it especially important for mining and obtaining chromium. Geoscientists claim there are nearly “11 billion tons” of chromite in the world, making it plentiful and abundant. With this amount of chromite available, renewable energy sources can be sustained for years.
Chromite and chromium are especially toxic to humans. Because of this, the process of mining chromite must be done very carefully. The iron-chromium alloys produce pollutants and dust, causing health risks to the environment and miners. It is important that geoscientists and energy corporations alike take these risks into account when mining chromite, to do so in a way that is safe and effective.
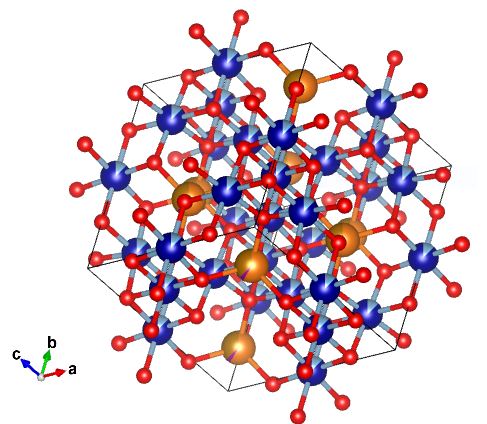
Chromite has a isometric crystal system. This means that the axes are both equal in length and perpendicular to one another (angles are 90°). The unit cell takes on a cubic form. To the left there is a picture of chromite's atomic makeup. There is oxygen (red), carbon (dark blue), and iron (orange/bronze).