

Monazite is a red/green-brownish, waxy mineral. It is hard and resistant. Monazite is formed when igneous rocks undergo crystallization and when clastic sedimentary rocks undergo metamorphism. It is commonly mined in placer deposits, which are masses of loose sediment mainly consisting of sand. Minerals that are often found with or near monazite are gold, platinum, magnetite, and zircon. These minerals are heavy and resistant, allowing them to withstand weathering and accumulate with monazite. Monazite consists of certain rare-earth elements that can be extracted through mining, such as neodymium.



| Color | Red-brown, green-yellow |
|---|---|
| Luster | Waxy |
| Streak | White |
| Hardness (Mohs) | 5.5 |
| Cleavage | Moderate | Specific Gravity | 4.6-5.4 |
| Chemical Formula | (Ce,La,Nd,Th)(PO4,SiO4) |
| Crystal Structure | Monoclinic |
| Classification | Phosphate |
Neodymium, one of the rare-earths that can be found in monazite, is a soft, silver, and metallic metal. Neodymium makes an extremely powerful magnet with its strong magnetic properties. Because of this, it is commonly used in engines, specifically wind turbines, for efficiency. Neodymium magnets allow wind turbines to be smaller and lighter, making them an ideal metal for renewable energy.



Monazite is a critical source for renewable energy because it contains a variety of rare-earth metals. Extracting these REE (rare-earth metals) can be done safely and they have the ability to be recycled as magnets, making them the optimal choice for wind energy and other generator systems.
Neodymium and other rare-earth metals can be separated from monazite through solvent extraction. Using a nonaqueous (not water-based solvent) solution such as Cyanex 923, a liquid phosphine oxide, scientists can extract neodymium from the mineral. This method is preferred, since it is a green solvent, making it environmentally friendly. Using a neutral extractant (Cyanex 923) shows efficiency over acidic extractants by avoiding chemicals used to strip the metal and unbalanced pH levels.
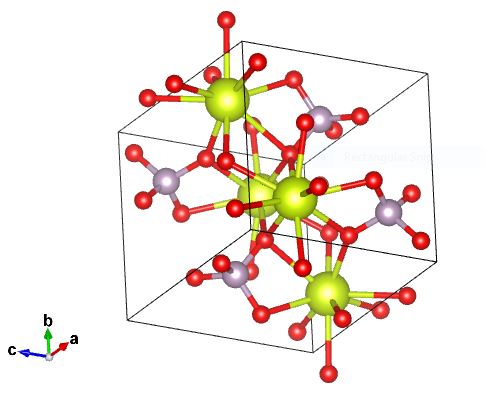
Monazite has a monoclinic crystal system. Each of the axes is unequal in length, while two are perpendicular to each other (angles are 90°) and one is not (angle is 103°). This forms a lopsided rectangular prism unit cell. To the left is a diagram of monazite's atomic makeup.There is oxygen (red), cerium (light blue), and phosphorus (lime green). The cerium can be substituted with either lanthanum, neodymium, or thorium, while the phosphorus can be substituted with silica.